Pharmacovigilance/Post-Marketing Surveillance
Pharmacovigilance
Pharmacovigilance is the science and activities relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with investigational products (IP) or any other medicine/vaccine related problem.
Goals of Pharmacovigilance:
• Continuous monitoring for risk-benefit ratio of investigational product (IP).
• Early detection of adverse events (AE) and interactions with other drugs/food.
• Identification of risk factors and mechanisms behind adverse events.
• Distribution of information about discovered AEs and interactions.
Steps of Pharmacovigilance:
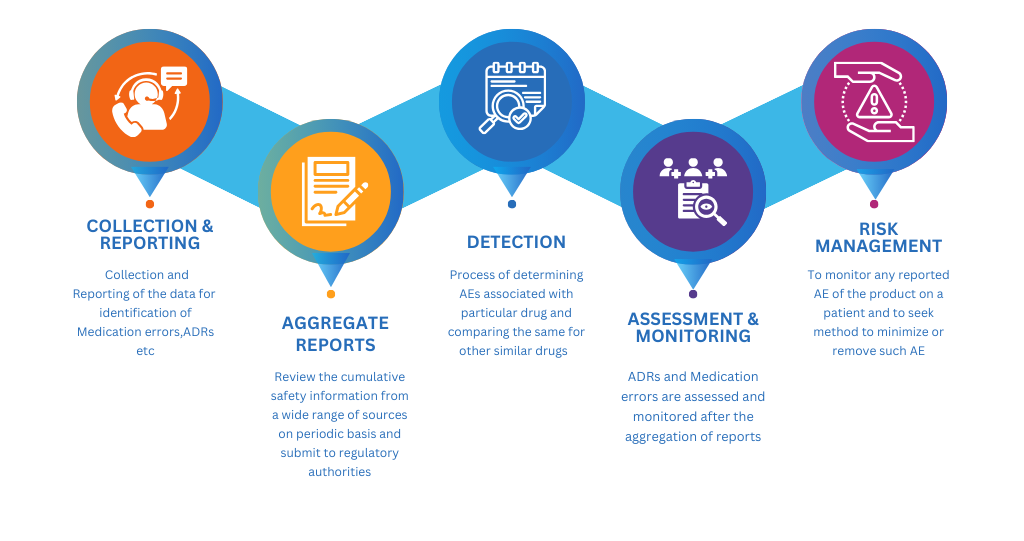
• Pharmacovigilance drives clinical trials that provide data on the risks and advantages of the drug and tries to discover whether the benefits exceed the risks; if they do, drug manufacturers take steps to obtain approval to market the new drug.
• PV analysis conducted in Phase I, Phase II, and Phase III clinical trials gives drug companies data on the drug’s safety profile. This data can be used for extra R&D if required or can be proposed to regulatory authorities to admit new markets to be obtained.
• After completing Phase III clinical trials and marketing authorization, the pharmaceutical company may conduct phase IV trials to monitor the drug on a much larger scale and in a less controlled real-world environment which is known as post marketing surveillance.
Post-Marketing Surveillance
The identification and collection of information regarding drugs after their approval for use in a population.
Implementing a sound post-market surveillance system based on principles of pharmacovigilance is essential.
The value of post-market surveillance to protect consumers defines and clarifies the professional regulatory role in communicating product safety as technology continues to increase data accessibility.
1. Ensuring Ongoing Safety
By continuously monitoring and evaluating the safety of a medical products, PMS helps ensure that patient safety is not compromised. It will identify potential safety issues associated with a product so any necessary corrective action can be taken.
2. Monitoring Effectiveness and Long-Term Outcomes
PMS will help assess a products effectiveness over its entire life cycle and evaluate its performance in a clinical setting. This information can be used to make improvements or changes in the future.
3. Identifying Unintended Adverse Events
PMS can help identify unintended adverse events associated, such as detecting issues that weren’t identified during clinical trials.
4. Improving the Efficacy of Existing Treatments and Medications
By collecting post-market surveillance data, medical products companies can better understand how their products interact with existing treatments and medications.
5. Improving Communication Between Stakeholders
PMS helps to facilitate better communication between medical products companies, healthcare providers, and patients. This is important for ensuring that all affected parties understand the risks associated with medical products and can work together to ensure patient safety.
CTU-RMI is an approved unit from DRAP for conducting of Phase-IV Clinical trials.
CTU-RMI is equipped to conduct the post marketing surveillance of medicinal products and medical devices.
CTU-RMI offers a trusted hospital setup for recruitment of large number of patients both from community and within hospital setting.
With its systematic and well-equipped unit, CTU-RMI offers a streamlined and proactive approach for collecting information.
CTU-RMI well organized communication system with appropriate tools and methods for investigating events occurred in PMS by connecting with patients directly.

