Our Expertise & Capabilities
As a Core Research Facility of Rehman Medical Institute, our primary objective is to provide comprehensive support to staff and collaborators for conducting a wide range of investigator-initiated trials spanning diverse therapeutic areas. Additionally, we extend our trial services to commercial clients, including the biotech, pharmaceutical, nutraceutical, and complementary medicine industries, as well as clinical research organisations.
Each trial within our portfolio is assigned to a specific CTU-RMI study team, taking into consideration the study’s size, type, and the level of collaboration agreed upon. The study team typically comprises a Manager of the Clinical Trials Unit, a Clinical Trials Coordinator, Clinical Research Associates (including both Physicians and Non-Physicians), and dedicated nurses equipped with phlebotomist skills. All of these team members are overseen by a Head of Department, ensuring seamless coordination and efficient functioning. On the operational front, our study teams are further supported by Electronic Data Capture team for CTU, which offers central database development, data management, and essential administrative assistance.
By maintaining a well-organised and proficient setup, we are committed to conducting successful trials and contributing to the advancement of medical knowledge across various fields. Our dedication to excellence ensures that both our internal research initiatives and collaborations with external partners are conducted with utmost professionalism and adherence to ethical standards.
Clinical Trials Unit-Rehman Medical Institute (CTU-RMI) has the following unique capabilities which outshines it among all the other sites across Pakistan:

Dedicated Reception Area
Waiting area for the hassle-free enrollment during the trials conduct.

Waiting Area
Dedicated reception area to receive research participants with private space for informed consent.
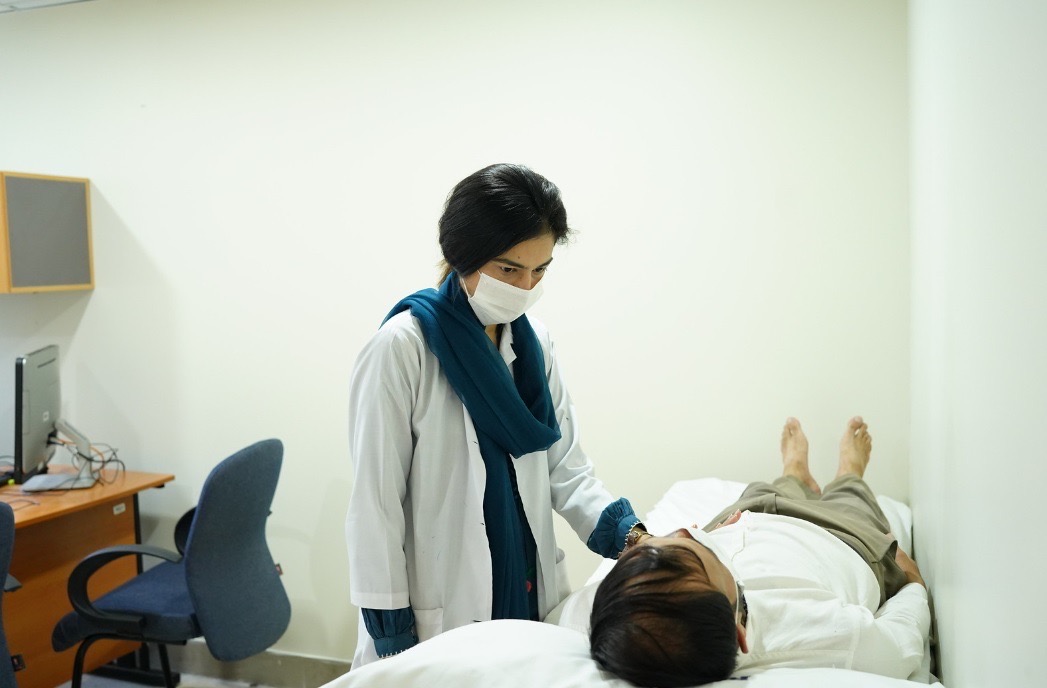
Assessment Rooms
Dedicated Assessment rooms.

IMP Administration
Dedicated IMP administration and observation room.

Document Storage Area
Research records and document storage area with secured access to the study personal only

Biometric Secured Archive
Biometric Archive Room for documents pertaining to clinical trial and study staff.
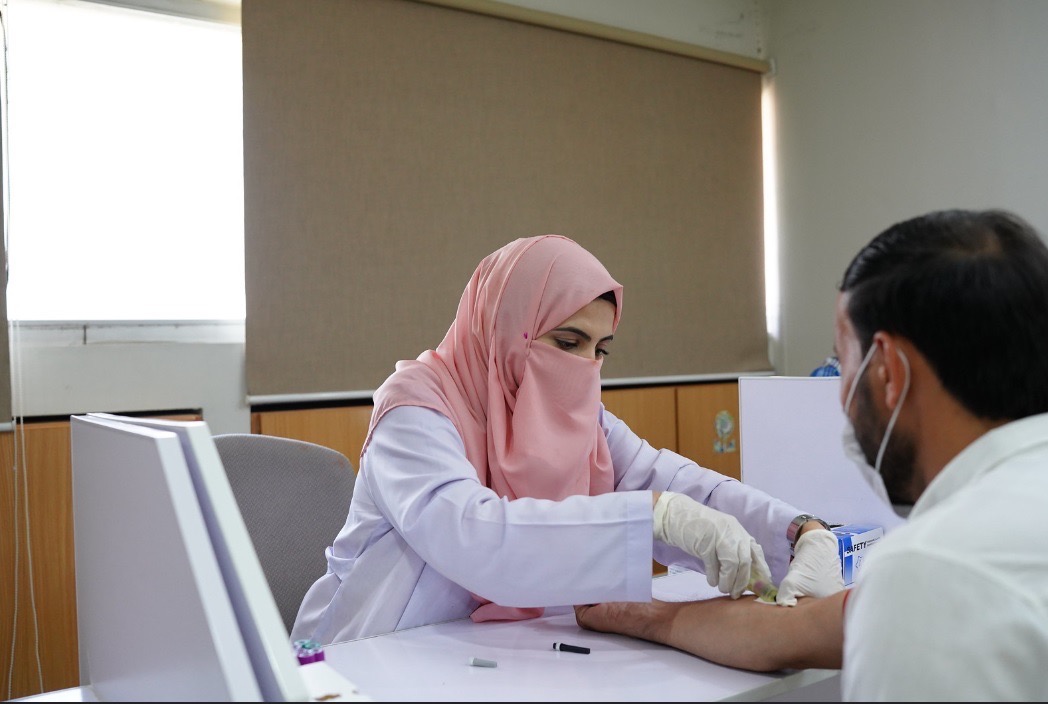
Phlebotomy & Sample Collection
Dedicated phlebotomy and sample collection room.
Welcome to RMI Clinical Trials Unit

Restricted Access to IVPB Room
Restricted access with thumb ID to authorized personnel only for any kind of sterile manufacturing(if needed).

Negative Pressure Area
Dedicated Negative Pressure area for any participants with infectious disease in order to minimize the risk for the site staff infection

HIPAA Compliant EMR
Access to medical records through HIPAA compliant EMR.

Dedicated CRA Secretariat
Dedicated Area for the site CRAs to ensure maintenance of the data effectively

Dedicated Call Center
Dedicated Call center team for: 1. Regular Patient concerns 2. Regular Follow ups 3. Any facilitation or support required during the recruitment of the trials

Dedicated Nursing Team
A specialised department focused on nursing care and services.

Dedicated Administration
Dedicated administration for the site management and trials facilitation during work.

Dedicated Finance Department
A specialized department responsible for financial management and accounting.

