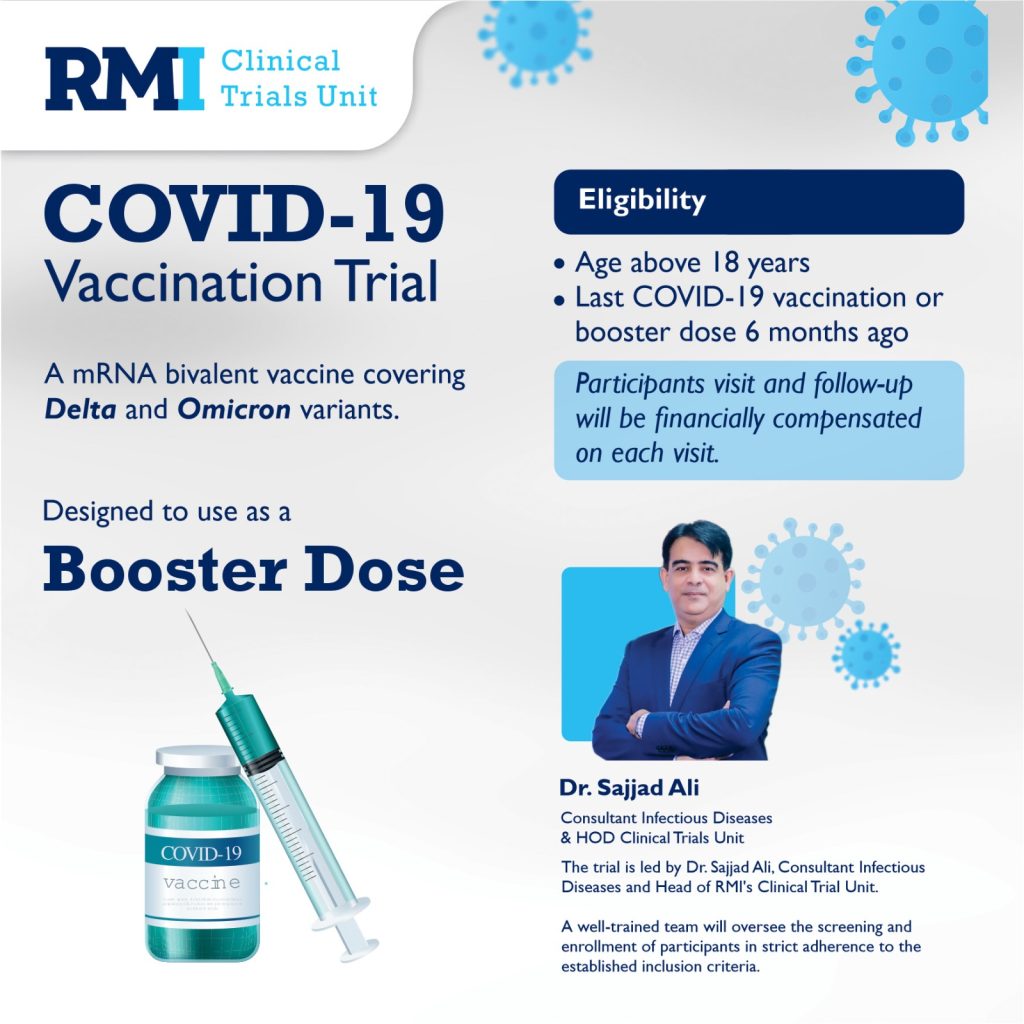
A Multi-center, Randomized, Blinded, Placebo-controlled, Phase 3 Clinical Study to Evaluate the Efficacy, Safety and Immunogenicity of SARS-CoV-2 Bivalent mRNA Vaccine (LVRNA021) as Booster in Participants Aged 18 Years and Older who Completed Primary/1 Booster Dose(s) of SARS-CoV-2 Vaccination
Study Status: Recruitment Completed
Thanks to All the Participants and their families for the active participation in the Trial